what happens to an atom radius when it gains an electron
Diminutive Radii
- Folio ID
- 610
Atomic radii is useful for determining many aspects of chemistry such as diverse physical and chemical properties. The periodic table profoundly assists in determining atomic radius and presents a number of trends.
Definition
Atomic radius is generally stated as being the total distance from an atom's nucleus to the outermost orbital of electron. In simpler terms, it can be defined equally something like to the radius of a circle, where the center of the circle is the nucleus and the outer edge of the circle is the outermost orbital of electron. As yous begin to motility across or downward the periodic table, trends emerge that help explain how atomic radii change.
The constructive nuclear charge (\(Z_{eff}\)) of an cantlet is the net positive charge felt by the valence electron. Some positive charge is shielded by the core electrons therefore the total positive charge is not felt by the valence electron. A detailed description of shielding and effective nuclear accuse tin can exist found here. \(Z_{eff}\) greatly affects the atomic size of an atom. So as the \(Z_{eff}\) decreases, the atomic radius will grow as a issue considering there is more than screening of the electrons from the nucleus, which decreases the allure between the nucleus and the electron. Since \(Z_{eff}\)decreases going down a group and right to left across the periodic table, the atomic radius will increment going down a group and correct to left across the periodic table.
Types of Radius with Respect to Types of Bonds
Determining the atomic radii is rather hard considering there is an dubiousness in the position of the outermost electron – we do not know exactly where the electron is. This phenomenon tin be explained past the Heisenberg Uncertainty Principle. To go a precise measurement of the radius, only still not an entirely correct measurement, we determine the radius based on the distance between the nuclei of two bonded atoms. The radii of atoms are therefore determined by the bonds they class. An atom will have different radii depending on the bond information technology forms; and so there is no fixed radius of an atom.
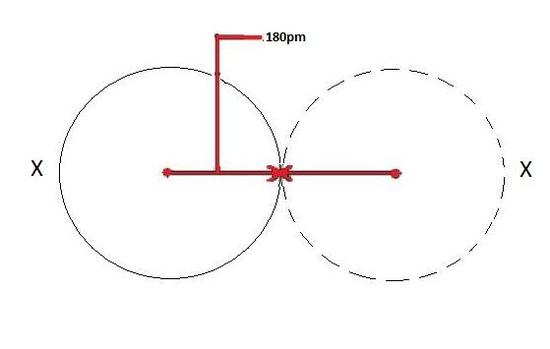
Covalent Radius
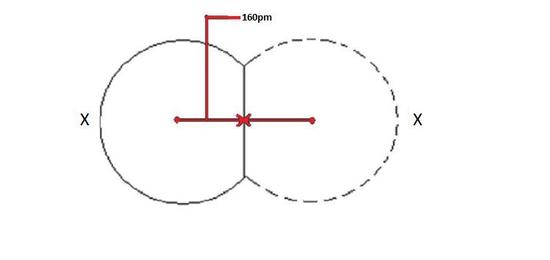
When a covalent bond is present between two atoms, the covalent radius can exist determined. When two atoms of the same element are covalently bonded, the radius of each atom will be half the altitude between the ii nuclei considering they equally attract the electrons. The distance between 2 nuclei will give the bore of an cantlet, but yous desire the radius which is half the diameter.
Covalent radii will increment in the aforementioned design as atomic radii. The reason for this trend is that the bigger the radii, the further the distance betwixt the two nuclei. See explanation for \(Z_{eff}\) for more details.
The covalent radius depicted beneath in Figure ane volition exist the same for both atoms because they are of the aforementioned chemical element as shown by X.
Ionic Radius
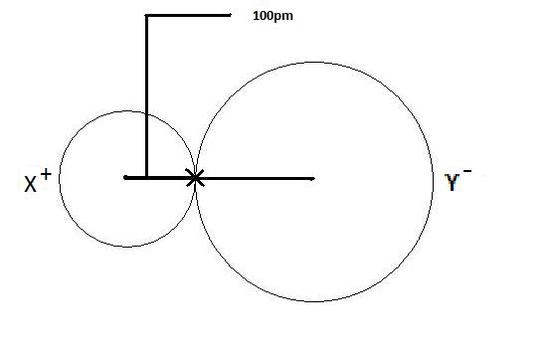
The ionic radius is the radius of an atom forming ionic bond or an ion. The radius of each atom in an ionic bond will be unlike than that in a covalent bond. This is an important concept. The reason for the variability in radius is due to the fact that the atoms in an ionic bond are of greatly different size. One of the atoms is a cation, which is smaller in size, and the other atom is an anion which is a lot larger in size. And then in order to account for this difference, one most get the total distance between the two nuclei and divide the altitude according to atomic size. The bigger the diminutive size, the larger radius information technology will have. This is depicted in Figure 2 as shown beneath where the cation is displayed on the left as 10+, and conspicuously has a smaller radius than the anion, which is depicted as Y- on the right.
Example 1: Cadmium Sulfide
If nosotros were able to decide the atomic radius of an atom from experimentation, say Se, which had an diminutive radius of 178 pm, then we could determine the diminutive radius of any other atom bonded to Se by subtracting the size of the atomic radius of Se from the full distance betwixt the two nuclei. And then, if we had the compound CaSe, which had a full distance of 278 pm between the nucleus of the Ca atom and Se atom, then the atomic radius of the Ca atom will exist 278 pm (total distance) - 178 pm (distance of Se), or 100 pm. This process can be applied to other examples of ionic radius.
Cations have smaller ionic radii than their neutral atoms. In contrast, anions have bigger ionic radii than their corresponding neutral atoms.
A detailed explanation is given below:
- The cation, which is an ion with a positive charge, by definition has fewer electrons than protons. The loss in an electron volition consequently event in a modify in diminutive radii in comparison to the neutral atom of interest (no charge).
- The loss of an electron means that there are at present more protons than electrons in the atom, which is stated above. This will cause a decrease in atomic size because there are now fewer electrons for the protons to pull towards the nucleus and will event in a stronger pull of the electrons towards the nucleus. It will as well decrease considering there are now less electrons in the outer crush, which will decrease the radius size.
- An analogy to this can be of a magnet and a metallic object. If 10 magnets and x metallic objects represent a neutral atom where the magnets are protons and the metallic objects are electrons, and so removing one metallic object, which is similar removing an electron, will cause the magnet to pull the metallic objects closer considering of a decrease in number of the metallic objects. This can similarly be said about the protons pulling the electrons closer to the nucleus, which as a upshot decreases atomic size.
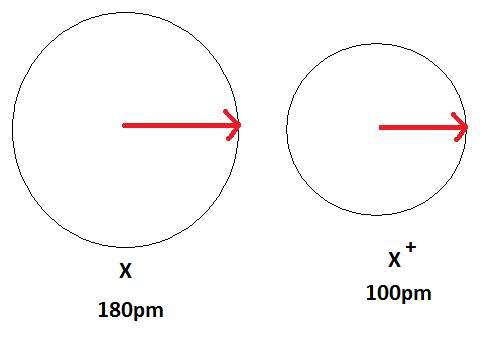
Figure 3 below depicts this process. A neutral atom Ten is shown here to have a bond length of 180 pm and so the cation 10+ is smaller with a bail length of 100 pm.
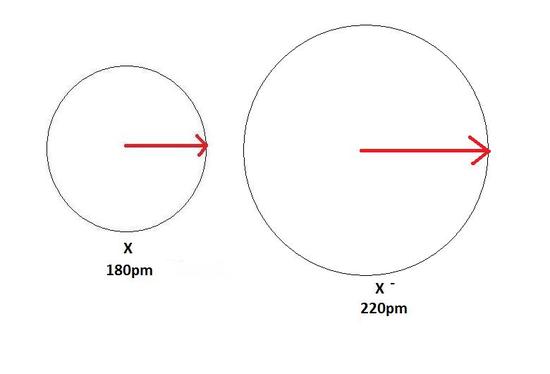
An anion, on the other hand, volition be bigger in size than that of the atom it was fabricated from considering of a gain of an electron. This can be seen in the Figure 4 below. The gain of an electron adds more electrons to the outermost shell which increases the radius considering there are now more electrons further away from the nucleus and at that place are more electrons to pull towards the nucleus so the pull becomes slightly weaker than of the neutral cantlet and causes an increase in diminutive radius.
Metallic Radius
The metallic radius is the radius of an atom joined by metallic bail. The metallic radius is half of the total altitude betwixt the nuclei of two next atoms in a metallic cluster. Since a metal will exist a grouping of atoms of the same chemical element, the altitude of each atom will exist the same (Effigy v).
Periodic Trends of Atomic Radius
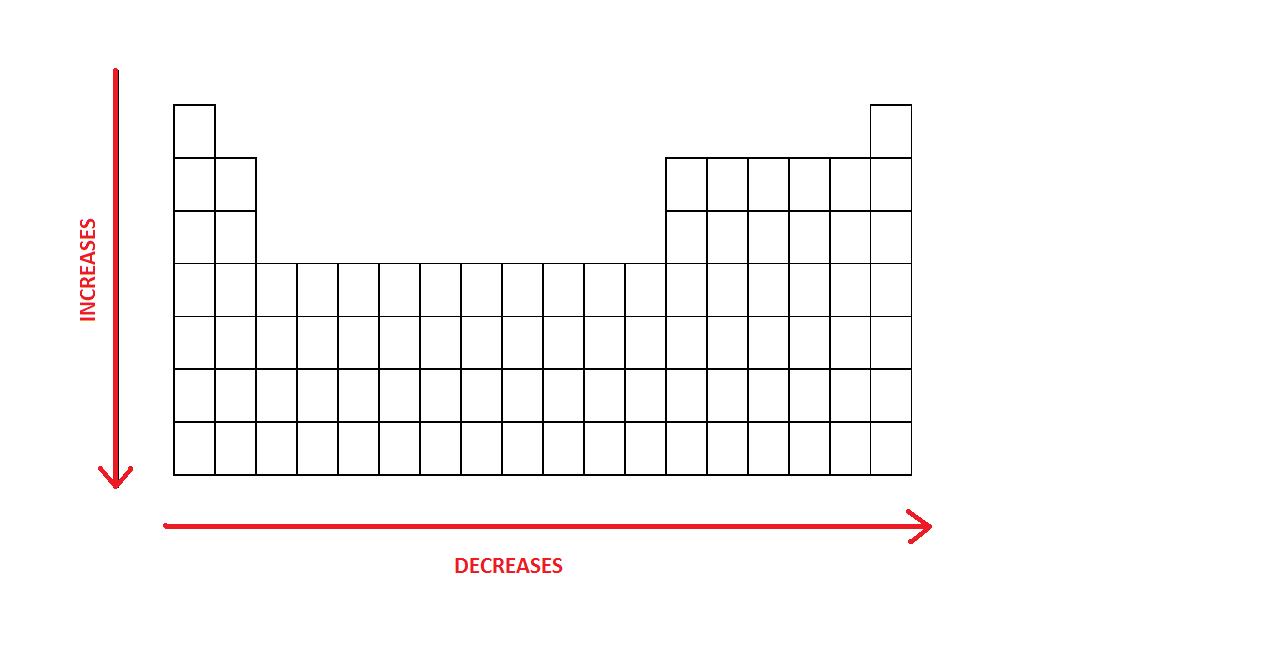
- An atom gets larger as the number of electronic shells increase; therefore the radius of atoms increases equally yous go down a sure group in the periodic table of elements.
- In general, the size of an atom volition subtract every bit you move from left to the right of a certain period.
Vertical Tendency
The radius of atoms increases as you lot get downwards a sure grouping.
Horizontal Trend
The size of an cantlet will decrease every bit you lot move from left to the correct of a catamenia.
EXCEPTIONS: Because the electrons added in the transition elements are added in the inner electron shell and at the same time, the outer trounce remains constant, the nucleus attracts the electrons inward. The electron configuration of the transition metals explains this phenomenon. This is why Ga is the aforementioned size as its preceding atom and why Sb is slightly bigger than Sn.
References
- Pauling, Linus. Atomic Radii and Interatomic Distances in Metal, Journal of the American Chemical Society 194769 (3), 542-553
- Petrucci, Ralph H., William South. Harwood, Geoffery F. Herring, and Jeffry D. Madura. General Chemistry. 9th ed. New Bailiwick of jersey: Pearsin Prentice Hall, 2007.
Problems
- Which atom is larger: One thousand or Br?
- Which cantlet is larger: Na or Cl?
- Which atom is smaller: Be or Ba?
- Which cantlet is larger: K+ or K?
- Put in order of largest to smallest: F, Ar, Sr, Cs.
- Which has a bigger atomic radius: Srtwo + or Se2 -?
- If Br has an ionic radius of 100 pm and the total altitude between K and Br in KBr is 150 pm, then what is the ionic radius of K?
- Which has a smaller atomic radius: Cs+ or Xe?
- If the distance between the nuclei of two atoms in a metallic bond is 180 pm, what is the atomic radius of 1 atom?
- If Z effective is increasing, is the atomic radius also increasing?
*Hint* When solving a radius-bail problem, identify the bond first and so utilise the standard method of finding the radius for that particular bond. Besides remember the trend for the atomic radii.
Answers
- M
- Na
- Be
- 1000
- Cs, Sr, Ar, F
- Se2 -
- fifty pm
- Cs+
- ninety pm
- No
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Atomic_Radii
0 Response to "what happens to an atom radius when it gains an electron"
Post a Comment